Zhongxin Jingwei, July 12 (Lin Yusi) With the increasing demand for HPV vaccine, the demand for HPV vaccine is in short supply due to limited production capacity, especially the nine-valent vaccine "one vaccine is hard to find", which makes many women who seek seedlings miss the best vaccination age.
Under this circumstance, recently, in some areas, the voice of "Nine-valent vaccine is about to relax the age limit to 45 years old" appeared. So, is it realistic to "age-extend" the nine-valent vaccine? How long will it take to achieve it?
Photo courtesy of nine-valent vaccine packaging box respondents
"selling contract packages" and "distributing goods" to sell nine-valent HPV vaccines
Cervical cancer is mainly caused by human papillomavirus (HPV). Wu Tao, an attending physician in the Department of Gynecology and Oncology of Shaanxi Cancer Hospital, told Zhongxin Jingwei that there are more than 100 kinds of HPV viruses, which are divided into high-risk and low-risk types. The common high-risk HPV types in China include 16, 18, 31, 33, 45, 52 and 58. Women who usually have sex may be infected with HPV virus, but most of them are transient infections. Like a cold, the body’s resistance is normal, and most of them can turn the virus negative by relying on their own immunity within one year. However, a small number of people with persistent high-risk human papillomavirus infection (generally believed to be more than one year) will lead to cervical precancerous lesions, which will continue to develop and even progress to cervical cancer.
However, cervical cancer can also be prevented, and the incidence of cervical cancer and precancerous lesions will be significantly reduced by vaccinating HPV vaccine among school-age women.
According to the annual report of Wan Tai Biology in 2021, according to the data of vaccine signing and wholesale by the Central Inspection Institute and local inspection institutes, from the perspective of large varieties, 181 batches of bivalent HPV vaccines were signed and wholesale, up by 269% year-on-year, and 44 batches of nine-valent HPV vaccines were issued, up by 83% year-on-year.
Even so, the nine-valent HPV vaccine is still "one seedling is hard to find" and even more sales tricks appear.
Recently, according to media reports, HPV vaccine needs to be "distributed", that is, with other vaccines or physical examinations, in order to get an HPV vaccine. A scalper who can help make an appointment for a nine-valent HPV vaccine told Zhongxin Jingwei that some hospitals have the situation of "selling contract packages", that is, compulsory physical examination, charging extra fees and bringing some profits to hospitals.
In addition, the scalper also revealed that because there are too many people who need to be vaccinated, releasing seedlings will never satisfy the market. In addition to releasing seedlings, some hospitals will buy and sell seedlings. The scalper said that he can help to make an appointment for the nine-valent seedlings by paying another 2,000 yuan, and he can make an appointment in about two weeks at the earliest. "All three needles can be made, and there is no need to renew the appointment. The needles are really nine-valent seedlings, and the traceability code on the box can be scanned to check the authenticity."

Nine-valent vaccination card in the new Jingwei Lin Yusi photo
Faced with the situation that the domestic HPV vaccine market is in short supply, there are also criminals who profit from smuggling nine-valent HPV vaccines at low prices. Recently, Liuzhou Intermediate People’s Court of Guangxi issued a first-instance verdict on a case of smuggling nine-valent cervical cancer vaccine. The defendant Xu Moli and other four people bought vaccines from Hong Kong at a low price and smuggled them to Shenzhen, and then sold them to various places. Four people smuggled 2,824 vaccines, involving nearly 3 million yuan, with a total profit of more than 120,000 yuan.
In fact, it is really helpless for people to turn to the yellow cattle to make an appointment and be asked by the hospital to "distribute" the seedlings. The way to make an appointment for HPV vaccines in various places is harsh, and it has been a long time since they were unable to "go ashore" for many years. Zhongxin Jingwei learned that Beijing made an appointment online and by telephone. However, many community hospitals have told Zhongxin Jingwei that the nine-valent HPV vaccine has no seedlings, and I don’t know when it will be available. Some hospitals in Beijing also have household registration restrictions, and people outside the jurisdiction are not allowed to vaccinate.
There are also areas such as Shenzhen that decide who can be vaccinated by "shaking the number". Many people have reported to Zhongxin Jingwei that the process of shaking the number is time-consuming and labor-intensive, and the speed of fighting hands is also luck. Many people can’t get it for half a year. Even if they can get it, there is no guarantee that the other two needles can be vaccinated on time, and there may be problems of overage.
Why is the nine-valent HPV vaccine still "one vaccine is hard to find"?
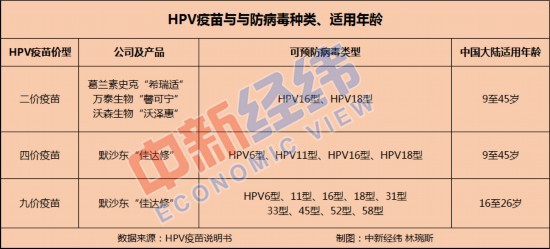
It is understood that there are currently three kinds of HPV vaccines listed in the world, and the number of HPV virus subtypes is different, including bivalent vaccine, tetravalent vaccine and nonavalent vaccine. Five HPV vaccine products have been approved for marketing in China, including three imported HPV vaccines and two domestic HPV vaccines.
In 2006, the world’s first HPV vaccine came out, until 2016, the first HPV vaccine approved for marketing in China — — The bivalent HPV vaccine "sirrah Shi" produced by GlaxoSmithKline has been in a blank period in China for ten years.
Luo Ling, the fund manager of Shanghai Congrong Investment Management Co., Ltd., said in an interview with Zhongxin Jingwei that the reason for the current strong demand is that many people have not been vaccinated with HPV before, and the demand has accumulated to be released at this stage. The next 5 to 10 years may be the stage of replanting the stock population, followed by the annual vaccination of the new population.
In 2018, the U.S. Merck nine-valent HPV vaccine "Jiadaxiu" was listed in mainland China, and Zhifei Bio became the exclusive agent of Merck HPV vaccine in China. However, up to now, the nine-valent HPV vaccine is still hard to find.
Behind the lack of seedlings, there is only one nine-valent vaccine manufacturer in Merck. Recently, Tian Anna, president of Merck China, pointed out in an interview with the media that it takes nearly four years for each dose of HPV vaccine to be produced and applied.
However, the supply of nine-valent HPV vaccine has also increased accordingly. Recently, Zhifei Bio said on the interactive platform that in the first quarter of 2022, the number of batches of nine-valent HPV vaccines increased by nearly 280% year-on-year.
Wu Tao said that vaccination with HPV vaccine is the best way to prevent HPV infection and cervical cancer, but don’t blindly wait and pursue the most expensive vaccine, but should be protected as soon as possible. If there is no nine-valent HPV vaccine, it is a better choice to vaccinate low-priced HPV vaccine.
In addition to the nine-valent HPV vaccine, the four-valent HPV vaccine market is currently in the hands of Merck. It is understood that the whole vaccination cost of tetravalent and nine-valent vaccines is 2394 yuan and 3894 yuan respectively. In the market competition of bivalent vaccine, domestic vaccine also gets a share with its price advantage.
In 2020, Wan Tai Bio’s bivalent HPV vaccine "Xinkening" went on the market, and one shot was only 329 yuan. Even if it was inoculated with three shots, it was only 987 yuan, far lower than the 1,740 yuan of the same dose of GlaxoSmithKline "sirrah Shi".
As the first domestic HPV vaccine, "Xinkening" adopts the research and development route of Escherichia coli technology, with low cost and high efficiency. According to the 2021 annual report, the biological business income of Wan Tai increased by 144.25%, mainly due to the increase of bivalent HPV vaccine, reagents and active raw materials, and the gross profit margin of vaccine business was as high as 92.55%. In 2021, the sales of bivalent HPV vaccine in Wan Tai exceeded 10 million, and the government procurement accounted for only 1.5%.
In March, 2022, watson biological bivalent HPV vaccine "Wozehui" was also approved for listing, and it was officially put on the market at the end of May, taking away part of the biological market in Wan Tai at a lower price.
At the end of May this year, the bivalent HPV vaccine of watson biological and Wanbiotai jointly won the bid for the procurement project of Jiangsu provincial government. The total purchase quantity is 32,000 pieces, of which 22,400 pieces were won at the proposed bid price of 246 yuan/piece in watson biological and 329 yuan/piece in Wan Tai Bio, only 9,600 pieces were won.
In this regard, Wan Tai Bio said that since its listing, its bivalent HPV vaccine has been mainly supplied to the self-funded market, and the purchase of free voluntary vaccination by local governments for specific age groups is relatively small, which has limited impact on the company’s operating income.
However, Wu Tao told Zhongxin Jingwei that there are regional differences in the distribution of cervical cancer in China, mainly concentrated in the central and western regions, and rural areas are highly prevalent in cities. Clinically, patients with advanced cervical cancer are mostly from rural areas. There are hundreds of millions of low-and middle-income women in China, who are concerned about the price of HPV vaccine besides the preventive effect and safety. "For economically underdeveloped people, the price will directly affect the choice of HPV vaccine types."
Is it close to relaxing the age limit?
On the track of nine-valent HPV vaccine, which is in short supply, there are more and more R&D and production enterprises in China. It is worth noting that some domestic nine-valent vaccines are expanding the age of the experimental population compared with the suitable age of 16 to 26 years approved by Merck nine-valent vaccine in Chinese mainland.
This may mean that with the listing of more nine-valent HPV vaccines in the future, not only can the situation of "one vaccine is hard to find" be alleviated, but the waiting school-age women do not have to be anxious because they are over 26 years old.
It is understood that Chongqing Bowei Baitai Biopharmaceutical Co., Ltd. (hereinafter referred to as Bowei Bio), Jiangsu Ruike Biotechnology Co., Ltd. (hereinafter referred to as Ruike Bio), Beijing Kangle Guardian Biotechnology Co., Ltd. and Wan Tai Bio’s nine-valent HPV vaccine have all entered the third clinical stage.
Wan Tai Bio started the Phase III clinical trial in September 2020. At that time, the company said that the phase III clinical trial of the nine-valent vaccine was aimed at evaluating the effectiveness and safety of the vaccine in female healthy volunteers aged 18-45. However, as early as 2020, Wan Tai Bio said that the nine-valent vaccine is not expected to be listed within three years according to clinical experience.
According to the information of National Medical Products Administration Drug Evaluation Center, the phase III clinical trial of nine-valent vaccine announced by Bowei Bio in 2020 also aims to evaluate the immunogenicity and safety of HPV vaccine among women aged 20-45 in China. Phase III clinical trial was completed in September 2021.
In June, 2021, Ruike Bio announced the effectiveness and safety of its nine-valent vaccine in the phase III clinical trial of healthy women, one of the purposes of which was to evaluate the safety and immunogenicity of three doses inoculated in healthy women aged 9-17, 16-26 and 18-45. However, the company did not disclose the completion date of the test.
Tao Lina, a vaccine expert, said in an interview with Zhongxin Jingwei that the duration of the third phase clinical trial needs to refer to the sample size: the larger the sample size and the higher the cost, the shorter the observation time; The smaller the sample size, the longer the observation time. You can’t have both fish and bear’s paw, with a large sample size and high investment. It will take one or two years to observe the third-phase clinical trial before you can see the significant difference between the vaccine group and the placebo group. The sample size is small, and it may take more than five or six years to get the difference of incidence and summarize it. It will take at least 2 to 6 years from the start of the third phase clinical trial of domestic HPV vaccine to the approval and listing.
Wu Tao told Zhongxin Jingwei that in 2019, Merck launched the third phase clinical trial of nine-valent HPV vaccine in China, bridging the immunogenicity and immune persistence test. The observation period is five years, that is, the earliest nine-valent HPV vaccine in China may relax the age limit to 45-year-old women by 2024. Men’s enrollment is also under way. However, if men in China want to get the nine-valent vaccine, they have to wait five years at the earliest.
However, Taulina also pointed out that relaxing to the age of 45 also means that more people are snapping up the nine-valent vaccine, which is still difficult to alleviate the situation of "one needle is hard to find" and even more intense. (Zhongxin Jingwei APP)
(The opinions in this article are for reference only, and do not constitute investment advice. Investment is risky, so you should be cautious when entering the market. )